|

 |
All homeopathic medicines are prepared under conditions strictly defined by the Homeopathic Pharmacopoeia of the United States (HPUS). The HPUS is a recognized Official Compendium, written into law in the Federal Food, Drug, and Cosmetic Act in 1938, and its current Revision Service (HPRS). Pharmacists should look to know that a manufactured product with HPUS indicated on its labeling provides them this assurance. |
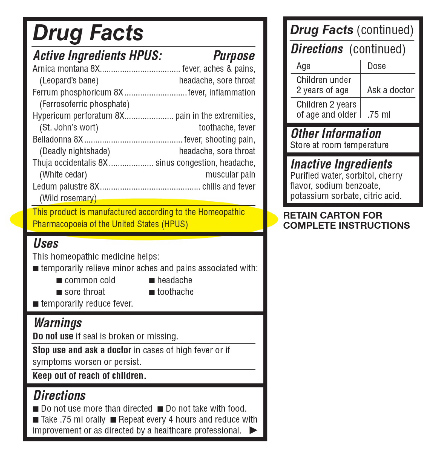
The U.S. Food and Drug Administration (FDA) does require that homeopathic remedies meet certain legal standards for strength, purity, and packaging. The labels on the remedies must include the following: at least one major indication (i.e., medical problem to be treated)
a list of ingredients
the dilution or concentration of the active substances
recommended dosage
mode of application
safety instructions
In addition, if a homeopathic remedy claims to treat a serious disease such as cancer, it needs to be sold by prescription. Only products for self-limiting conditions (minor health problems like a cold or headache that go away on their own) can be sold without a prescription.
|
|
|
 |

|

